12. It's all there in black and white

How many different materials can you burn to make black pigments? What does the most important white pigment in Western art have to do with horse poop? It’s all there in black and white.
Lead white in manure: the ‘Dutch process’
Lead white has been used as a pigment for thousands of years, and until recently was the only white pigment with excellent opacity (covering power), durability, fast drying, and good handling properties. Vermeer used lead white to paint white highlights, and mixed it with other colours to make them paler.
Where did Vermeer’s lead white come from? First we have to find out where the raw material – lead ore – comes from. It was dug from the earth at one of several lead ore deposits in Europe. Each one contains a specific type of lead. Lead isotope analysis can help determine whether Vermeer’s lead white came from a northern or southern European source. Within the Girl in the Spotlight project, Paolo D’Imporzano and Gareth R. Davies from VU Amsterdam carried out lead isotope analysis on samples from the Girl. They confirmed that the types of lead in the ground and paint are similar, and came from a lead source that is consistent with other Northern European paintings. They are currently comparing these to the data from other paintings by Vermeer.

Lead white has been made in roughly the same way for thousands of years, but apparently the Dutch were so good at it that it became known as the ‘Dutch stack process’. They poured vinegar into clay pots and suspended rolled-up sheets of lead above the liquid. They stacked the pots in a shed, surrounded by horse manure. As the manure fermented, it released heat and carbon dioxide (and a nasty smell!). This caused a chemical reaction that formed a crust of basic lead carbonate. They washed this flaky white crust, dried it and ground it up to a powder, which could then be used as a pigment.
Lead in the Girl
Just like x-rays of the human body, x-rays of paintings show differences in absorption of the radiation by different materials. The differences depend on the thickness of the materials, and the atomic weight of the elements in them. Lead white appears white on an x-ray because it is ‘heavy’: i.e. it has a high atomic weight.
Since lead white is a component of the ground, the x-ray shows marks where the ground was spread over the canvas using a knife.
Paint that contains a lot of lead white are also x-ray opaque: her eyes, the highlight on the pearl, her shirt, the highlights in her headscarf, and the left side of her face. The Girl’s face contains a lot of lead white where she faces the light. This creates a strong contrast between the lit and shadow areas, which reinforces its 3-dimensionality.

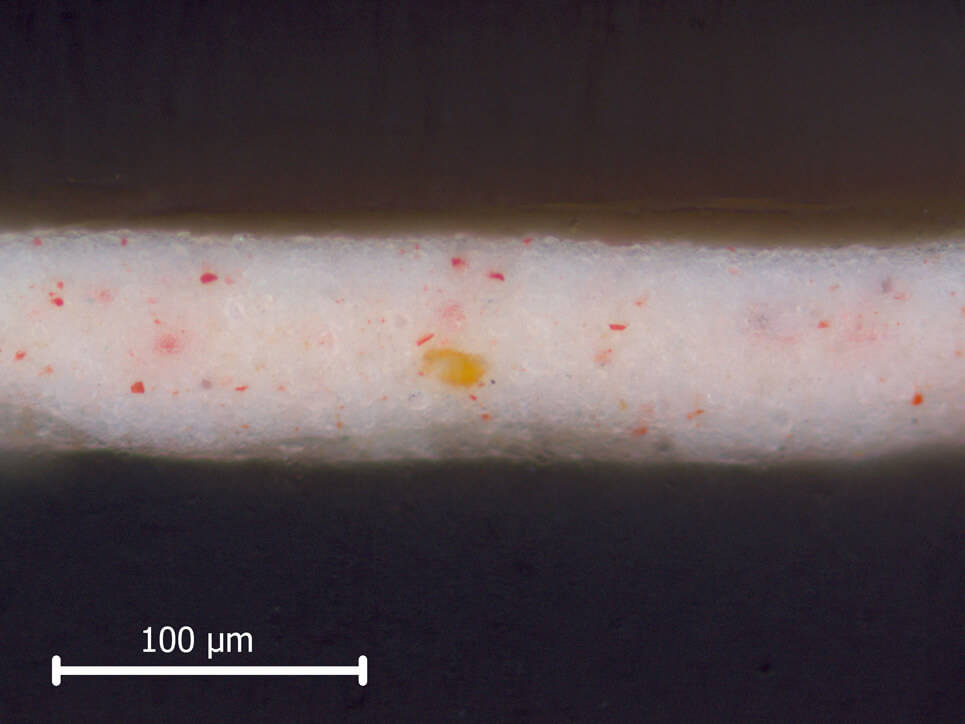

A cross-section of the paint from the Girl’s skin shows that it is mostly made up of lead white particles, with some red and brown pigments in it. When the sample is examined with SEM/EDX, the backscatter image shows the lead particles clearly; because they are ‘heavy’, they appear bright white.
As black as she is painted
Vermeer seems to have used two different black pigments: charcoal and bone black. Sometimes he mixed them together. Perhaps he was trying to achieve a specific colour. Charcoal produces a bluish black, whereas bone black is brownish. The black pigments that Vermeer used in the Girl are mostly hidden beneath the surface, or mixed with other colours. As you’ll find out in a blog in a couple of days, the background of the Girl wasn’t originally meant to be black.
Charcoal is made by charring wood (usually willow or grape vines) inside a closed vessel in a fire. A charcoal stick can be used for drawing, but can also be crushed and mixed with a binding medium to make ink or paint.
[fig. 12e: sample 21 with charcoal]
This cross-section is from the background of the Girl, where Vermeer applied charcoal black as an underlayer beneath a translucent glaze. The splintery black particles are the charred wood fibres.
The other black pigment that Vermeer used was bone black. What is it made from? Bones, of course…but also animal horns or ivory. Because bones are mostly made of calcium phosphate, the elements calcium (Ca) and phosphorus (P) can be detected with the scientific techniques SEM/EDX and FIB-TEM. These particles are also usually rounder and smaller than charcoal splinters.
Vermeer used a mixture of charcoal and bone black in the underlayers beneath the clothing (which I’ll discuss in a later blog post).

Because charcoal and bone black are made from organic materials (wood and bone), they both contain carbon. Carbon-containing layers beneath the surface of the painting can be detected by using infrared examination.
References
- Natural Pigments, Stack Process White Lead: Historical Method of Manufacture
- Fabian, Daniel and Giuseppino Fortunato (2010) ‘Tracing White: A Study of Lead White Pigments found in Seventeenth-Century Paintings using High Precision Lead Isotope Abundance Ratios.’ In: Trade in Artists’ Materials: Markets and commerce in Europe to 1700, Archetype, London, pp. 426-443.
- Winter, John and Elisabeth West FitzHugh (2007) ‘Pigments based on carbon.’ In: Artists’ Pigments: A handbook of their history and characteristics, Barbara H. Berrie (ed.), Vol. 4, National Gallery of Art, Washington and Archetype, London, pp. 1-37.
Acknowledgements
- Lead isotope analysis: Paolo D’Imporzano and Gareth R. Davies (Department of Earth Sciences, VU Amsterdam). They are involved in the NICAS project ‘Multi-isotopic analysis of early modern art (MITEEMA).’
- SEM-EDX and microscopy: Annelies van Loon (Mauritshuis / Rijksmuseum)
- SEM-EDX and FIB-TEM: Ralph Haswell (Shell Technology Centre Amsterdam)
- Near-infrared imaging: John Delaney and Kate Dooley (National Gallery of Art, Washington)
- Infrared photography: René Gerritsen (René Gerritsen Art & Research Photography)
